Niche construction in ecology
What is a niche?
A key feature of modern niche concepts is that the ecological niche is defined relative to the organism at its centre. This is because organisms determine which environmental factors are significant components of their world, a perspective very much in line with niche construction theory.
Organisms actively modify their niches, and those of other organisms, through their activities and choices. However, niches have typically been construed as static rather than evolving. In order to devise a more dynamic niche concept, Odling-Smee et al. (2003) characterize an evolutionary niche as ‘the sum of all the natural selection pressures to which the population is exposed’, an approach well-received by ecologists (Chase & Leibold 2003). The evolutionary niche can be viewed as a dynamic (i.e. evolving) version of the more familiar ecological niche when the latter is characterized as an n-dimensional hypervolume of those conditions and resources relevant to the population (Hutchinson 1957).
How do organisms modify niches?
Organisms change their niches, by physically changing environments, or by relocating in space, thereby exposing themselves to new conditions. They may create novel niches by inceptive niche construction, by innovating. For example, the evolution of improved paper technology in Polistinae wasps apparently had massive effects on the geographic distribution, colony size and social complexity of these wasps (Hansell 1993).
Organisms can also buffer out some of the changes in their environments that are caused by other agents by counteractive niche construction. For instance, wasps regulate temperature in their nests by bringing droplets of water to cool the nest, or by stretching and contracting their abdomens in unison to warm the nest.
Through niche construction, organisms can influence and control some flows of energy and materials among trophically interconnected organisms, even without being part of those flows themselves. The modification of natural selection by niche construction may be direct, as occurs when animals construct artifacts, such as nests or dams. Conversely, organisms may only modify natural selection indirectly, or gradually, for instance through the slow accumulation in their environments of the by-products of their metabolisms, and detritus producing activities – which generates an ecological inheritance.
How does niche construction relate to ecosystem engineering?
Niche construction theory explores two interacting processes. The first is the modification of niches by the environment-altering and -selecting activities of organisms. The second is the subsequent evolution of one or more populations in response to selection altered by the first process. The environment-altering aspect of niche construction is broadly similar to the concept of ecosystem engineeringThe modification by organisms of physical surroundings (e.g., light environment, physical habitat structure) so as to modulate the availability of resources or energy fluxes in an ecosystem in ecology (Jones et al. 1994, 1997).

Jones et al. drew attention to the comparative lack of ecological research dedicated to studying organisms that modulate the availability of resources and habitats in ecosystems. For instance, beavers’ dams have dramatic effects on the flows of energy and matter through river environments. Kelp are another example: their growth creates forests that provide habitat for countless other organisms.

Many species do influence energy flows, mass flows, and trophic patterns in ecosystems, by generating ‘engineering’ control webs based on mosaics of connectivity among species . Jones et al. have shown that engineering webs make significant contributions to the regulation of energy and matter flows in ecosystems, alongside webs of trophic interactions.
How are ecologists using niche construction theory?
Recent years have witnessed considerable efforts to operationalize the niche construction concept within ecology, and devise means for its investigation. These methods provide tools to explore how niche construction triggers ecological and evolutionary feedbacks, to detect the ecological signatures that it leaves, and to explore its effects on biodiversity.
Post & Palkovacs (2009) illustrate how niche construction can generate eco-evolutionary feedbacks, and how these can be investigated.

Odling-Smee et al. (2013) describe some of the ecological and evolutionary impacts on ecosystems of niche construction.
Matthews et al. (2014) propose an operational framework to evaluate comparative and experimental evidence of the evolutionary consequences of niche construction, and suggest how such research can improve our understanding of ecological and evolutionary dynamics in ecosystems .
Brathen & Raivolainen (2015) show that species sharing a single trait or species belonging to a growth form can act as collective niche constructors, and be important predictors of species diversity in ecological communities. In tundra plant communities, forbs and grasses were the least abundant growth forms, yet they were the strongest positive predictors of species diversity.
Ware et al. (2019) propose a conceptual model that shows how feedbacks across levels of organization link theory associated with eco-evolutionary dynamics, niche construction and the geographic mosaic theory of evolution.

Coexistence, range expansion and adaptive radiation
Theoretical studies show that niche construction has important effects on species coexistence, range expansion and adaptive radiation.
Kylafis & Loreau (2011) introduce the concept of ‘ecological niche construction’, the process whereby an organism improves its environment to enhance its growth and persistence, which they argue is an important missing element of niche theory. In a model of two consumers that compete for one limiting resource and one predator, they show how niche construction modifies the traditional niche-deteriorating impacts of its agent or of competing species, and hence the potential for species coexistence. Thus niche construction can promote mutualism and niche partitioning, although under different conditions it can strengthen interspecific competition. The authors also show how niche construction strongly affects the realized niche of a species.
Kylafis & Loreau (2008) demonstrate that niche construction can generate local spatial effects that can allow a population to promote its own range expansion. In this manner, niche construction potentially plays a role in adaptive radiation. Such effects can link eco-evolutionary feedbacks to the process of adaptive radiation. Rather than lineages simply diversifying to “fill” available niches, niches themselves may be diversifying (Erwin 2005), a process subsequently termed “self-propagating adaptive radiation.”
Krakauer, Page, & Erwin (2009) show that niche construction can drive coevolutionary events, exacerbate and ameliorate competition, affect the likelihood of coexistence and produce macroevolutionary trends.
Key readings
Buser CC, Newcomb RD, Gaskett, AC & Goddard MR. 2014. Niche construction initiates the evolution of mutualistic interactions. Ecology letters. 17(10): 1257-1264. Demonstrates experimentally how through niche construction (modification of fruit) the yeast Saccharomyces cerevisiae attracts Drosophila, facilitating its propagation.
Erwin DH . 2005. Seeds of diversity. Science. 308:1752–1753. Presents the hypothesis that organisms construct new niches, leading to diversification.
Hamblin SR, White PA, Tanaka MM. 2014. Viral niche construction alters hosts and ecosystems at multiple scales. Trends in Ecology & Evolution. 29(11): 594-9. Viruses modify host environments, and these modifications drive evolutionary feedback between the virus and its environment across multiple scales from cells to ecosystems.
Hansell MH. 1993. The ecological impact of animal nests and burrows. Functional Ecology. 7: 5–12. Provides extensive empirical examples of the ecological impact of niche construction through nest and burrow construction.
Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos. 69: 373-386. The most authoritative introduction to the concept of ecosystem engineering.
Krakauer DC, Page KM, Erwin DH. 2009. Diversity, dilemmas, and monopolies of niche construction. American Naturalist. 173: 26–40. Demonstrates a fundamental dilemma of niche construction, whereby the construction of a shared resource leads to a tragedy of the commons, with competition tending to eliminate niche construction strategies.
Kylafis G, Loreau M. 2008 Ecological and evolutionary consequences of niche construction for its agent. Ecology Letters. 11: 1072-1081. This theoretical analysis shows that this niche construction allows the persistence of the plants under infertile soil conditions that would otherwise lead to their extinction.
Kylafis G, Loreau M. 2011 Niche construction in the light of niche theory. Ecology Letters. 14: 82–90. Introduces the concept of ‘ecological niche construction’, the process whereby an organism improves its environment to enhance its growth and persistence.
Matthews B, De Meester L, Jones CG, Iberlings BW, Bouma TJ, Nuutinen V, van der Koppel J & Odling-Smee J. 2014. Under niche construction: an operational bridge between ecology, evolution and ecosystem science. Ecological Monographs. 84.2: 245–263. Written for professional ecologists with a view to operationalizing niche construction, this article illustrates how niche construction can be investigated.
Mumby, PJ, van Woesik R. 2014. Consequence of ecological, evolutionary and biogeochemical uncertainty for coral reef responses to climatic stress. Current Biology. 24: R413–R423. Describes how niche construction and ecological inheritance by corals impacts on how they respond to human-generated changes in conditions, including climate change.
Odling-Smee FJ, Erwin D, Palkovacs E, Feldman M, Laland KN. 2013 Niche construction theory: a practical guide for ecologists. Quarterly Review Biology. 88, 3-28. This article provides a practical guide to how niche construction theory can be deployed by ecologists and evolutionary biologists to explore eco-evolutionary dynamics.
Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Philosophical Transactions of the Royal Society B. 364: 1629–1640. Illustrates how niche construction contributes to eco-evolutionary dynamics.
Sultan SE. 2015. Organism & environment: Ecological development, niche construction, and adaptation. Oxford: Oxford University Press. The most up-to-date and authoritative and comprehensive treatment of niche construction, packed with empirical examples, particularly in plants and animals.
Contents
Niche construction in the human sciences
Niche construction theory has had a particular impact in the human sciences, including biological anthropology (Anton et al. 2013; Zeder 2018), archaeology (Smith 2007; O'Brien & Laland 2012), and psychology (Flynn et al. 2013).
Niche construction is also widely recognized to have played important roles in human evolution (Fuentes 2009, 2017; Kendal et al. 2011; Anton et al. 2014), the evolution of human cognition and language (Bickerton 2009; Laland 2017), human impacts on biodiversity (Ellis 2015; Boivin et al. 2016), and the origins of domestication and agriculture (Smith 2007; Zeder 2015).
Why has niche construction theory been so influential in the human sciences? One reason is that it is self-apparent that humans possess an unusually potent capability to regulate, construct and destroy environments, and that this is generating some pressing current problems (e.g. climate change, …deforestation, urbanization). A second reason is niche construction theory’s recognition of human agency (and of human activities guiding selection rather than merely resulting from it), which is attractive to human scientists (Odling-Smee et al. 2003; Kendal et al. 2011; O’Brien & Laland 2012), with the important caveat that this emphasis on agency does not imply human niche construction is necessarily conscious or deliberate. A third reason is that niche construction theory emphasizes how acquired characters play an evolutionary role, and this is particularly relevant to human evolution, where our species appears to have engaged in extensive environmental modification through cultural practices (Laland et al. 2010). Niche construction theory provides an evolutionary framework capable of accommodating human cultural behavior in a way that doesn’t diminish its complexity and uniqueness, yet also shows how these behaviors are an extension of general evolutionary processes that pertain to non-human organisms.
Such cultural activities are typically not themselves biological adaptations (rather, they are the adaptive product of those much more general adaptations, such as the ability to learn, particularly from others, to teach, to use language, and so forth, that underlie human culture) and hence, cannot accurately be described as extended phenotypes.
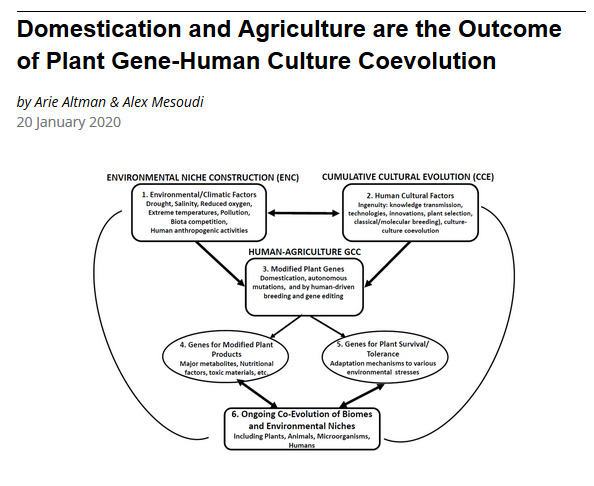
Mathematical models reveal that niche construction due to human cultural processes can be as potent as gene-based niche construction, and establish that cultural niche construction can modify selection on human genes and drive evolutionary events (Laland et al. 2001; Creanza et al. 2012; Creanza & Feldman 2014, 2016). This interaction, known as ‘gene-culture co-evolution’, is also found in some animals, such as killer whales, orangutans and reed warblers (Whitehead et al. 2019). However, gene-culture coevolution reaches its zenith in humans. Cultural change will typically occur faster than genetic adaptation, and this has allowed cultural niche construction to play a prominent role in human evolution (Creanza et al. 2017).

There is now little doubt that human cultural niche construction has co-directed human evolution (Laland et al. 2010; Creanza et al. 2017). Humans have modified selection, for instance, by dispersing into new environments with different climatic regimes, devising agricultural practices or domesticating livestock.
The best-researched example of gene-culture co-evolution is the finding that dairy farming created the selection pressure that led to the spread of alleles for adult lactase persistence (Feldman & Cavalli-Sforza 1989; Gerbault et al. 2011). However, analyses of the human genome have identified many hundreds of genes subject to recent selection, many in response to human cultural activities (Laland et al. 2010). The evolution of lactose persistence may be representative of a very general pattern of gene-culture coevolution over the last 20,000 years.
One study reported 27 separate genes, known to have been subject to recent selection, for which the inferred cultural selection pressure is a change in diet associated with the advent of agriculture (Laland et al. 2010). In addition to the breakdown of dairy products, the list also includes genes expressed in the metabolism of carbohydrates, starch, proteins, lipids, phosphates, plant secondary compounds and alcohol, as well as jaw muscle fibres and tooth-enamel thickness.
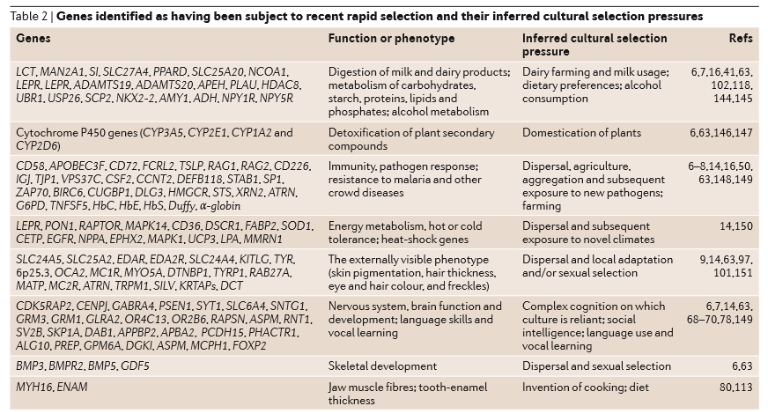
In some West African populations, the cultural niche construction practice of cultivating yams has inadvertently generated selection for the haemoglobin S allele that underlies sickle cell anaemia. Slash and burn agriculture creates conditions that lead to increased standing water when it rains, and these puddles are perfect breeding grounds for malaria carrying mosquitos. The enhanced incidence of malaria, in turn, generates selection for gene variants that confer resistance to malaria, one of which is Hb S.
Cultural niche construction can also feed back to influence the cultural evolution of a second trait, for instance allowing for the coevolution of marriage customs and sex ratio biases (Creanza et al. 2012). Here, the spread of a cultural trait creates a cultural niche in which another cultural trait flourishes. A good example is provided by the demographic transition. The reduction in birth rate during the demographic transition is often viewed as a paradox because, from a traditional evolutionary perspective, it is difficult to envisage why individuals should prefer to have fewer children. However, cultural niche construction models show that if a cultural norm favoring greater education spreads, a preference for smaller family size follows, allowing the fertility rate to drop (Ihara & Feldman 2004).
The primary cause of cultural complexity is another conundrum that niche construction theory has helped to resolve. The issue had proven contentious, with some researchers suggesting that large populations support diverse cultural knowledge and others arguing that environmental factors are more important. The paradox is resolved when niche construction is taken into account (Collard et al. 2012; Fogarty & Creanza 2017). The effects of a changeable environment, to which food gatherers are subject, can be attenuated by the niche-constructing activities of food-producers, such as agriculturalists. As a result, the effect of the environment on cultural complexity is stronger in food-gathering populations and weaker in food-producing populations, whilst the effect of population size is stronger in producers compared to gatherers.
The niche-construction perspective has been productive in many other studies of human social behavior (Kendal et al. 2011; Creanza et al. 2017), including some that describe some dramatically large-scale consequences of culturally driven change. These include the extinction of megafauna after the arrival of humans and consequent shaping of global species distributions (Boivin et al. 2016), and the striking changes of landscapes in Bali (Lansing et al. 2009; Lansing & Fox 2011) and Polynesia (Quintus & Cochrane 2018) with complex cascades of ecological and social consequences following the cultivation of crops.
The human transition from hunting and gathering to food production economies provides another illustration of the explanatory power of niche construction theory (Smith 2007a, 2007b, 2012, 2016). Archaeological and paleo-environmental records from eastern North America, Amazonia, the Near East, and, increasingly, China,contradict the assumption of traditional explanatory frameworks that environments change and species adapt (Smith 2011; Zeder 2017; Ren et al. 2016; Lombardo et al. 2020). Conversely, explanations based on niche construction theory are well-supported in these regions. Archaeologists have concluded that extensive data concerning the domestication of plants and animals, and the development of agricultural societies, supports predictions derived from niche construction theory (Zeder & Smith 2009; Zeder 2017, 2018; Piperno 2017). Initial domestication of plants and animals, in turn, offer proponents of niche construction theory and the EES an opportunity to evaluate core assumptions about niche construction, ecological inheritance, plasticity first evolution, and reciprocal causality, in a major transition with profound impact on both human and non-human organisms (Zeder 2018).
Psychologists and linguists are also using niche construction theory. Researchers have stressed that the human mind is a symbol-generating and artefact-devising system, as a result of which children develop in a rich human-constructed world teeming with diverse symbols and artefacts. The constructed cognitive niche both shapes, and is shaped by, the child’s learning and development (Flynn et al. 2013). Peterson et al (2018) argue that physical signs and symbols left in the environment have played an important role in human evolution.
Niche construction is also now central to several accounts of how language evolved. For instance, Bickerton (2009) describes how our ancestors constructed scavenging niches that required them to communicate in order to recruit sufficient individuals to drive off predators away from megafauna corpses. He maintains that our use of language, in turn, created a new niche in which sophisticated cognition was beneficial. Tomlinson (2015, 2018) makes a related argument, and shows how it explains the origins of human music.
Key readings
Anton SC, Potts R, Aiello LC. 2014. Evolution of early Homo: An integrated biological perspective. Science. 345(6192): 1236828. A recent overview of current thinking in the field of human evolution. Niche construction is thought to play a central role.
Bickerton D. 2009. Adam’s Tongue. How humans made language, how language made humans. New York: Hill & Wang. A readable account of the evolution of language from a leading authority in which niche construction plays a central role.
Boivin NL, Zeder MA, Fuller DQ, Crowther A, Larson G, et al. 2016. Ecological consequences of human niche construction: examining long-term anthropogenic shaping of global species distributions. Proceedings of the National Academy of Sciences USA. 113(23): 6388-96. Argues that niche construction has been a key feature of human evolution.
Creanza N, Kolodny O, Feldman MW. 2017. Cultural evolutionary theory: How culture evolves and why it matters. Proceedings of the National Academy of Sciences USA. 114:7782-7789. Discusses how cultural niche construction has codirected human evolution.
Flynn EG, Laland KN, Kendal RK, Kendal JR. 2013 Developmental niche construction. Developmental Science. 16(2): 296-313. Draws attention to parallels between niche construction theory and some fields of developmental science, and illustrates now the niche-construction perspective can be fruitfully applied within developmental psychology.
Fogarty L, Creanza N. 2017. The niche construction of cultural complexity: interactions between innovations, population size and the environment. Philosophical Transactions Royal Society B. 372(1735): 20160428. Shows how niche construction modules the impact of the environment on cultural complexity.
Gerbault P, Liebert A, Itan Y, Powell A, Currat M, Burger J, Swallow DS, Thomas MG 2011. Evolution of lactase persistence: an example of human niche construction. Phil. Trans. R. Soc. B. 366: 863–877. The domestication of cattle and consumption of dairy products is a compelling human example of niche construction, which has selected for alleles for adult lactose absorption.
Kendal JR, Tehrani JJ & Odling-Smee FJ. 2011. Human niche construction. Philosophical Transactions of the Royal Society B. 366: 1566. A series of articles on human niche construction.
Laland KN, Odling-Smee FJ, Myles S. 2010. How culture shaped the human genome: Bringing genetics and the human sciences together. Nature Reviews Genetics 11: 137–148. Reviews the evidence for gene-culture co-evolution in humans.
O’Brien M, Laland KN. 2012. Genes, culture and agriculture: an example of human niche construction. Current Anthropology. 53: 434-470. Draws on niche-construction theory and gene-culture co-evolutionary theory to propose a broad theoretical framework with which archaeologists and anthropologists can explore human co-evolutionary dynamics.
Zeder MA. 2012. The broad spectrum revolution at 40: resource diversity, intensification, and an alternative to optimal foraging explanations. Journal of Anthropological Archaeology, 31(3):241-264. Discusses a key role for human niche construction in the origin of agriculture.
Niche construction in developmental biology
Niche construction arises in development: it occurs through the activities of individual organisms during their lifetime (ontogeny) as they modify environmental states, and has ecological and evolutionary consequences when those individual-based local effects scale up across populations, and over time. By modifying their local environment, organisms can affect their own future development, whilst the construction of nests and other ‘nursery’ environments can shape and regulate the conditions experienced by developing offspring. For these reasons, niche construction theory is explicit about the need to treat niche construction as both a developmental process and a cause of evolution (Odling-Smee et al. 2003).
There exists a complementarity between the fields of niche construction and ecological developmental biology (Laland et al. 2008). Niche construction emphasizes the ability of the organism to modify the environment to form a supportive niche, whilst ecological developmental biology emphasizes the ability of environmental agents (e.g. temperature, density, symbionts) to modify the developing organism.
Animal and plant development provide some of the most direct examples of niche construction (Gilbert 2020). Here, two modes of niche construction can occur, often simultaneously. First, the developing organism can modify its environment to construct niches for its own development. Second, the organism can become the niche for other organisms that will later alter it. Both of these modes involve developmental plasticity–the ability of an organism to change its phenotype in response to the presence of environmental agents.
Developing organisms modify their own environments to construct niches
The formation of galls by gall wasps and the formation of the placenta by mammalian embryos are examples of how organisms construct their own developmental environment. The female goldenrod gallfly lays its eggs on the goldenrod. When they hatch, the caterpillars eat the goldenrod stem, and their salivary proteins induce cell proliferation in the goldenrod, triggering formation of a gall. The larva enters the gall, which becomes its home, and continues eating from within. As winter approaches, the larva begins to produce sugars that act as an antifreeze. The trigger for this synthesis is not temperature but aromatic substances produced by the desiccating gall (Williams & Lee, 2005). Here, we see both reciprocal induction on ecological level and reciprocal causation at evolutionary level. The gallfly larva creates a niche by causing the plant to change its development, and that niche provides safety, nutrition, and the cue for the larva to change its development as winter approaches.

Mammalian development provides another example. Mammalian embryos construct their niche by instructing the uterus to alter its cell cycles, its adhesion proteins, and by inducing the formation of blood vessels. The fetus induces changes in the uterus preparing it for implantation of an embryo, thereby causing the uterus to become a habitat for the developing organism. In return, the uterus reciprocally helps induce the formation of the placental tissues of the embryo (Spencer et al. 2004; Ticconi et al. 2006).
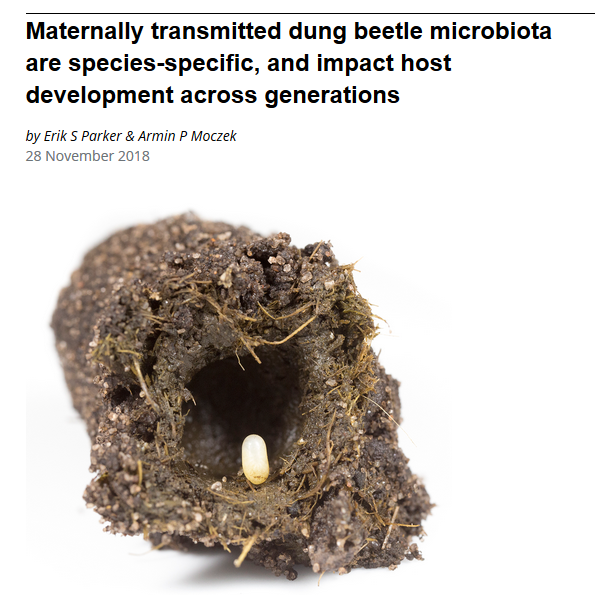
Niche construction during development can also be the product of behavior. For instance, female dung beetles manufacture and bury a brood ball of dung and insert into it a faecal pedestal onto which they lay an egg. Through this niche construction they provide a safe home, food supply and microbiome for their developing young (Schwab et al. 2016). The developing larvae also engages in niche construction, in a manner that affects its development. It processes the brood ball in ways that propagate and change the composition of the microbiome. Experiments show that both maternal and offspring niche construction strongly affect offspring size, fitness and trait characteristics, such as sexual dimorphism (Schwab et al. 2016, 2017). This example illustrates how niche construction can both shape developmental trajectories and affect evolutionary outcomes. Ongoing work is exploring how beetle populations diverge in their reliance on certain forms of niche construction, and how it affects range expansions and reproductive isolation.
Organisms construct their environment and alter each other
One of the most exciting areas of developmental niche construction concerns symbiotic bacteria. Here, the bacteria are both part of the environment and an organism seeking a niche. Bacteria and developing animal are environments for each other, and scaffold each other’s development (Chiu and Gilbert 2015). Bacteria are an essential component of the inheritance system in many insects and vertebrates. For instance, Dedeine et al. (2001) found that females of the wasp Asobara tabida cannot make their oocytes without products being made from the Wolbachia bacteria stored in them: A. tabida treated with antibiotics were unable to produce mature eggs.
Similarly, mammalian development is not complete without signals from symbiotic bacteria (Hooper et al. 2001; Xu and Gordon 2003). Mice bred without gut bacteria have aberrant digestive systems and defective immune systems. The bacteria induce gene expression in intestinal epithelia, and these genes are responsible for activating the pathways that allow intestinal capillaries to form and lipids to be transported (Hooper et al. 2001; Stappenbeck et al. 2002). Without these microbes, mice lack the capillary vasculature of the intestinal villi.
Ley et al. (2006) have shown that human babies acquire their gut microbial communities from the vagina and the feces of their mothers early in life. Babies born through Caesarian section had an altered colonization pattern compared with vaginally delivered babies.
Niche construction by symbionts has been studied extensively in the squid Euprymna scolopes and the luminescent bacterium Vibrio fischeri (McFall‐Ngai & Ruby, 1991; Montgomery & McFall‐Ngai, 1994). The adult squid is equipped with a light organ composed of sacs filled with light‐emitting bacteria, but newly hatched squid have neither the bacteria, nor the light organ to house them. Rather, the symbiotic bacteria interact with the larval squid to build their niche. The juvenile squid acquires the bacteria from seawater by pumping through its mantle cavity (Nyholm et al. 2000). The bacteria bind to a ciliated epithelium in this cavity, and secrete chemicals that induce hundreds of genes in the epithelium to become active, leading to the differentiation of the surrounding cells into storage sacs for the bacteria, and the expression of genes encoding opsins and other visual proteins in the light organ (Chun et al. 2008; Koropatnick et al. 2004; McFall‐Ngai. 2008; Tong et al. 2009). In this mutualistic arrangement, both organisms change their gene expression patterns, in a beneficial way. The bacteria get a niche, and the squid develops a light organ that allows it to swim at night.

Another interesting case of bacterial niche construction concerns the formation of the rumen of ruminants such as cattle, sheep and deer. Newborn calves have sterile rumens, and the digestive tube becomes colonized by microbes as the calf pass through the birth canal. When the calf starts eating solid food, bacteria produce plant-wall‐digesting enzymes that metabolize the polysaccharides. Over 70% of the cow’s energy comes from this microbial digestion of plant fiber (La Reau & Suen, 2018). The bacteria in the rumen multiply when given this food, and as they proliferate, they produce volatile fatty acids, that cause the rumen to grow and complexify (Gilbert, 2020). Here, the gut bacteria help construct their niche, the rumen, without which the cattle could not survive.
Many experiments now show that symbionts are not merely passive travelers, but provide essential functions for their hosts. In mammals, it has been estimated that 50-90% of cells are symbionts (Bäckhed et al. 2005; Sender et al. 2016). For instance, about one-third of the metabolites in our blood are derived from bacteria, whilst symbionts have been shown to be critical for normal brain development, and effective functioning of the immune system (Gilbert & Epel 2009; Gilbert et al. 2012). The bacteria of the gut are critical in the organogenesis of the gut capillaries and lymphoid tissues (Round et al. 2010).
Microbiomes (symbionts such as bacteria, archaea, protists, fungi and viruses) can be transmitted from one generation to the next, including via eggs and seeds, through the birth canal in mammals, or by drinking mother’s milk (Gilbert et al. 2012; Roughgarden et al. 2018). Many organisms rely on their microbiome to carry out essential processes: for example, corals rely on microalgae for photosynthesis and energy production, termites rely on intestinal protists to digest cellulose, and legumes require rhizobial bacteria for nitrogen fixation (Kamra 2005; Gilbert et al. 2012; Roughgarden et al. 2018).
“Development is a multi-species project” (Chiu & Gilbert 2015), and microbial communities do not merely “occupy” hosts but rather host and microbiome “are constantly constructing and modifying each other as local niches” (Chiu & Gilbert 2015).
Extra-genetic inheritance and the ‘start-up niche’.
The inheritance of symbionts from the mother is an example of extra-genetic inheritance. Recent years have witnessed the accumulation of empirical support for extra-genetic inheritance through multiple pathways, including epigenetic, ecological, behavioral, and cultural inheritance systems and the transmitted microbiome (Bird 2002; Jablonka & Lamb 2005; Bonduriansky & Day 2018). Diverse resources are transmitted from parents to offspring, including components of the egg, hormones, symbionts, epigenetic marks, prions, small RNAs, antibodies, ecological resources and learned knowledge.
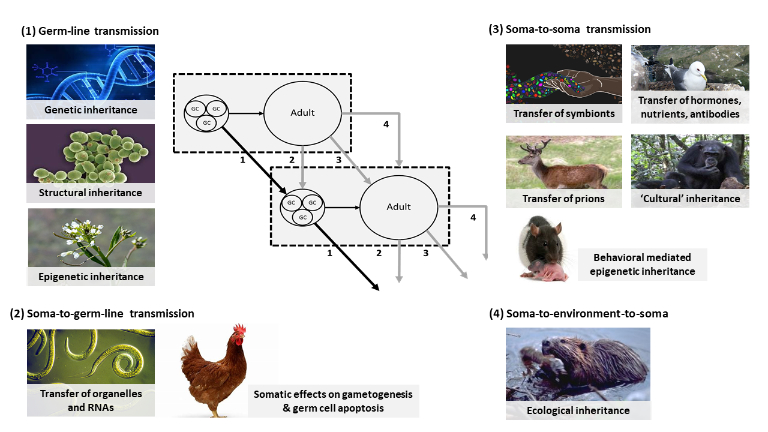
Traditionally considered proximate causes of development, it is now evident that some of these factors can lead to the inheritance of phenotypes, and many researchers now attempt to integrate these components into an extended concept of heredity (Bonduriansky & Day 2018; Danchin et al. 2011; Jablonka & Lamb 2014). These additional pathways allow for ‘soft’ inheritance (environmental influences on heredity). Recognition of extra-genetic inheritance has important implications, both for understanding development and comprehending how development can influence evolution.
Offspring inherit not just genes but a ‘start-up niche’, comprising a specific parentally chosen location for birth and a transmitted package of resources (Odling-Smee 2010). For example, phytophagous insects typically choose to lay eggs on specific host plants, which become food for their larvae, whilst in birds, nutrients and hormones are provided in the yolk for embryonic nutrition. Many organisms provide protective chemicals for their offspring in this start-up niche, including antibodies such that the young can survive before their immune systems mature (birds, mammals), compounds that are poisonous or distasteful to predators (moths) (Dussourd et al. 1988), or even sun-blocks that protect transparent embryos and larvae from the effects of solar radiation (Goldstone et al. 2006).
From the niche-construction perspective, the key task for any developing organism becomes the active regulation of its inherited ‘niche’, by responding to its environment, and by altering its environment, in ways that keep its personal organism–environment relationship continuously adaptive, for the rest of its life. This active ‘niche regulation’ undermines the (generally assumed) independence of environments from developing organisms: just like selective environments, developmental environments are not independent on the organism. Niche construction provides a pathway through which developmental processes can influence evolutionary processes.
Many researchers have argued that development plays significant, but not yet fully appreciated, evolutionary roles (e.g. Gould & Lewontin 1979; West-Eberhard 2003). For instance, micro- and macro-evolutionary patterns are commonly viewed as shaped by developmental biases and constraints (Jablonski 2020; Jackson 2020), while developmental plasticity is perceived to provide phenotypic variants that can be later stabilized by the natural selection of genetic variation (Gilbert et al. 2015; Uller et al. 2020; Levis & Pfennig 2020). Genes can be ‘followers, not leaders’ in evolution (West-Eberhard 2003). Niche construction contributes to these mechanisms (Laland et al. 2008; Hall 2012; Chiu & Gilbert 2015; Roughgarden et al. 2018).
Key readings
FJ, Gilbert SF. 2008. EvoDevo and niche construction: building bridges. Journal of Experimental Zoology Part B. 310:549–566. Points to common ground between niche construction and evo devo. Argues that the same conceptual barriers have hindered both.
Chiu L, Gilbert SF. 2015. The birth of the holobiont: multi-species birthing through mutual scaffolding and niche construction. Biosemiotics. 8: 191-210.
Schwab DB, Casasa S, Moczek, AP. 2017. Evidence of developmental niche construction in dung beetles: effects on growth, scaling and reproductive success. Ecology Letters. 20, 1353–1363.
Schwab DB, Riggs HE, Newton ILG, Moczek AP. 2016. Developmental and ecological benefits of the maternally transmitted microbiota in a dung beetle. The American Naturalist. 188(6): 679-82
Niche construction in medicine
Human niche-constructing activities may inadvertently promote disease. Cultural practices, such as cultivating crops and keeping animals, appear to have aided the spread of diseases, such as malaria and sickle-cell anaemia, leading to the selection of alleles that confer resistance to the promoted diseases (Durham 1991; Laland et al. 2010; O’Brien & Laland 2012).
Many human genes that provide some immunity from, or resistance to, disease or pathogens are now known to have been subject to recent selection, and are thought to have been promoted by agriculture or other farming practices (Laland et al. 2010).
The inadvertent construction of disease niches by human cultural activities may have been going on for some time, and is implicated in the emergence of tuberculosis, an ancient human disease. The controlled use of fire may have triggered the spread of TB, through a combination of increased opportunities for transmission brought about by the developing social culture that fire use encouraged and the lung damage that smoke inhalation caused (Chisolm et al. 2016).

Another system that exhibits feedback between human cultural niche construction and genetic selection is the host-parasite relationship between antibiotic treatment and viability selection for antibiotic-resistant bacterial strains. This is an example of inter-specific cultural niche construction. Boni and Feldman (2005) found that the cultural transmission of antibiotic use favours selection of resistant bacterial strains, which in turn can result in cultural selection for the avoidance of antibiotic use.
This kind of host behaviour can result in the classic niche-construction phenomenon of maintaining strain polymorphism even in parameter regions where it would not otherwise be expected. Interestingly, the evolution of either the host activity or the parasite strain can be viewed as a niche-constructive activity that modifies the selective environment of the other. Potentially this promotes an arms race between two types of transmitted information, cultural and genetic.
Here, the niche constructive effects can be described fully in terms of trait–trait co-evolution as there is no ecological inheritance of a constructed ‘resource’ that is separate from the cultural or the genetic information transmission systems. The relative frequencies of bacterial resistance and sensitivity are the effective ‘resource’ influencing the cultural evolution of antibiotic treatment, and visa versa.
Disease vectors too can construct niches. For instance, as obligate parasites, viruses too are constantly modifying the host environment, and these modifications are now thought to drive evolutionary feedback between the virus and its host, across multiple scales, from cells to ecosystems (Hamblin et al. 2014). For instance, the rabies virus causes aggression of the host and thereby facilitates its own propagation.
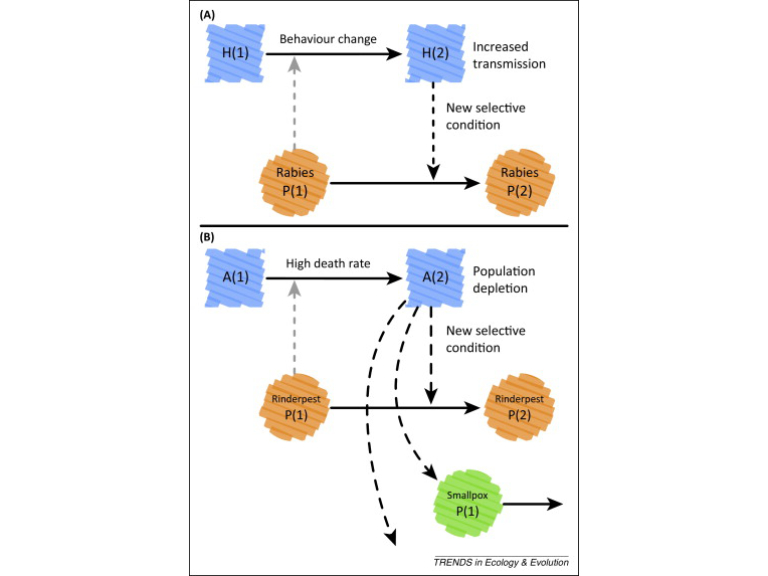
Key readings
Boni MF, Feldman MW. 2005. Evolution of antibiotic resistance by human and bacterial niche construction. Evolution. 59(3): 477–491. Explores coevolution of medicines and bacterial strains through reciprocal niche construction.
Chisholm RH, Trauer JM, Curnoe D, Tanaka MM. 2016. Controlled fire use in early humans might have triggered the evolutionary emergence of tuberculosis. PNAS. 113(32):9051–56. Discusses how controlled use of fire may have led to spread of tubercolosis
Hamblin SR, White PA, Tanaka MM. 2014. Viral niche construction alters hosts and ecosystems at multiple scales. Trends in Ecology & Evolution. 29(11): 594-9. Viruses modify host environments, and these modifications drive evolutionary feedback between the virus and its environment across multiple scales from cells to ecosystems.
Niche construction and conservation biology
The impact of niche construction on diversity
Organisms do considerably more in ecosystems than compete with and eat each other (trophic interactions). They also produce, modify, and destroy habitat and resources, in the process driving co-evolution and regulating hydrological, nutrient, and element (e.g., carbon) cycling (Odling-Smee et al. 2003). Through niche construction and ecosystem engineering, organisms create ‘engineering’ control webs that affect the stability and productivity of ecosystems (Jones et al. 1994, 1997).
Across scales that encompass both the presence and absence of ecosystem engineering/niche construction, the net effect should be to enhance species richness via a net increase in habitat diversity (Jones et al. 1997). Recent studies provide support for this hypothesis. For example, natural sites with and without beavers exhibit low overlap in species composition. By increasing habitat heterogeneity, beavers increased herbaceous plant species numbers by more than 33% (Wright et al. 2002).
Another example is seaside arrowgrass (Triglochin maritima), which facilitates plant diversity in salt marshes by creating elevated rings (maintained structurally by its rhizomes) with increased reductive potentials and reduced salinity. T. maritima supports both a greater abundance of species and the growth of species not present in the adjacent substratum (Fogel et al. 2004).
Brathen & Raivolainen (2015) show that in tundra plant communities, forbs and grasses were the least abundant growth forms, yet they had a strong positive effect on species diversity through their effects on ecosystem process rates and nutrient cycling. The effects of these plants are completely disproportionate to their share of biomass.
Niche construction and conservation strategies
Findings like these have important implications for understanding, managing, and conserving ecosystems (Crain & Bertness 2006; Boogert et al. 2006; Laland & Boogert 2010). Academics and politicians alike have failed to consider fully both the important role that these control webs play in ecosystems, and the consequences of human activities that destroy those webs of connectance.
If ecosystems are threaded by ‘engineering’ control webs, then the disappearance of key niche constructors may lead to abrupt changes in the resources and selection created by them, greatly affecting other species. Populations that have become dependent on engineered habitat and resources may be unable to cope with the loss, while genetic adaptation across generations is often too slow to counteract environmental modifications, leading to further declines in biodiversity and ecosystem functioning. This highlights the importance of preserving species that construct or maintain habitat and resources for other species (Crain & Bertness 2006; Boogert et al. 2006; Ehlers et al. 2008).
One might avoid unforeseen negative consequences of introducing engineering species by replenishing the niche constructors’ effects on the environment, rather than the organisms themselves (Odling-Smee et al. 2003). Some examples of the mimicking of engineering effects include introduction of artificial mussel mats (Crooks & Khim 1999), artificially created leaf ties otherwise produced by caterpillars (Lill & Marquis 2003), and relocalization of natural structures to provide lizard refuges (Pringle 2008). Research is required to explore the extent to which these manipulations can be successful, affordable and feasible at scales relevant to conservation goals.
Boogert et al. (2006) suggest an implementation strategy to conserve ecosystems through the conservation of niche-constructing activities. The strategy includes the following steps:
Step 1) Set conservation goals for the target ecosystem.
Step 2) Determine the key engineers in the target ecosystem, which will often require additional research.
Step 3) Conduct pilot studies to explore which of the following procedures might be most feasible and effective: (a) enhancing key engineers’ current activity by introducing more conspecifics or providing them with the resources required for population growth, (b) enhancing key engineering activities by introducing different species that engineer in the same manner, (c) introducing artificially manufactured products of the key engineers, and (d) creating optimal levels of abiotic and biotic factors to facilitate key engineers through trophic or nontrophic links. When the ecosystem is negatively affected by invading engineers, one could investigate the effectiveness of equivalent steps to reduce their impact.
Step 4) Implement the optimal engineering strategy or combination of strategies on a small scale. Follow up by monitoring and assessment.
Step 5) If step 4) has the desired outcome, implement the successful engineering strategy on a large scale.

Niche construction and the Anthropocene
Unlike any other species in Earth’s history, human niche construction is so potent that we have gained the capacity to transform the functioning of an entire planet (Ellis 2015; Waters et al. 2016).
Though contemporary rates and scales of anthropogenic environmental change are unprecedented, human societies began transforming Earth’s ecology many thousands of years ago (Smith & Zeder 2013; Boivin et al. 2016).
Anthroecology theory holds that human societies scaled up and gained the capacity to transform Earth’s functioning through a long-term evolutionary process, in which increasing societal scales and increasingly transformative ecosystem engineering are coupled through positive feedbacks (known as ‘runaway sociocultural niche construction’) (Ellis 2015, Ellis 2018).

Much attention has been given to how human activities can have a negative impact on biodiversity, and rightly so, but it is important to recognize that human impacts have multitudinous, complex and often conflicting effects on the biosphere. Counter-intuitively, recent research shows that human niche construction is actually increasing biodiversity in restricted contexts. For instance, it is known that a complex ecosystem of attached organisms develops on submerged structures (such as oil rigs) in the marine environment, which supports a localized food web that could not exist without them (Fortune & Paterson, 2018). The impact of human activity on the environment needs to be assessed on a case-by-case basis, drawing on careful science.

Key readings
Boogert NJ, Laland KN, Paterson DM. 2006. The implications of niche construction and ecosystem engineering for conservation biology. Bioscience. 56: 570–578. Discusses the implications of niche construction theory for understanding, managing and conserving ecosystems.
Bråthen KA, Raivolainen VT. 2015. Niche construction by growth forms is as strong a predictor of species diversity as environmental gradients. Journal of Ecology. 103: 701–713. Shows how certain plants affect species diversity.
Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos. 69: 373-386. The most authoritative introduction to the concept of ecosystem engineering.
Wright JP, Jones CJ, Flecker AS. 2002. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia. 132: 96-101. Shows how beaver niche construction increases biodiversity